In today’s highly regulated and competitive industries, especially within the Life Sciences and Manufacturing sectors, maintaining impeccable quality standards is non-negotiable. Preventive action, particularly through an effective CAPA (Corrective and Preventive Action) program, plays a pivotal role in identifying and mitigating potential issues before they escalate. Recognizing the signs that your team requires preventive action can save your organization from costly recalls, regulatory fines, and reputational damage.
Understanding CAPA and Its Importance
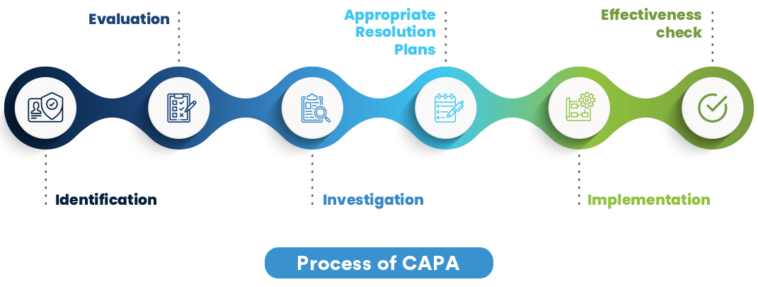
What is CAPA?
CAPA stands for Corrective and Preventive Action, a systematic approach used to identify, investigate, and address issues within an organization. It not only focuses on correcting existing problems but also on implementing preventive measures to avoid recurrence.
The Role of a CAPA Program in Quality Management
A well-structured CAPA program is integral to Quality Management Systems (QMS) as it ensures continuous improvement and compliance with industry regulations. By proactively addressing potential issues, companies can maintain high standards of quality and efficiency.
Identifying Early Warning Signs for Preventive Action
Frequent Deviations and Non-Conformances
One of the primary indicators that your team needs preventive action is the occurrence of frequent deviations and non-conformances. These recurring issues suggest underlying systemic problems that need to be addressed through a comprehensive CAPA program.
Increased Customer Complaints and Returns
A surge in customer complaints and product returns is a clear sign that there are flaws in your processes or products. Implementing change control measures within your CAPA program can help identify and rectify these issues promptly.
Monitoring Change Control in Pharma
The Significance of Change Control in Pharma
In the pharmaceutical industry, change control is critical to ensuring product quality and compliance. Effective change control in pharma involves rigorous evaluation and documentation of any modifications to processes, equipment, or materials.
Integrating Change Control into Your CAPA Program
Incorporating change control into your CAPA program ensures that all changes are systematically reviewed and approved, minimizing the risk of adverse effects on product quality and regulatory compliance.
Analyzing Data Trends and Metrics
Utilizing Data to Predict Potential Issues
Data analysis is a powerful tool for predicting and preventing future problems. By analyzing trends and metrics, you can identify patterns that indicate the need for preventive action.
Implementing Data-Driven CAPA Programs
A data-driven CAPA program leverages quantitative insights to make informed decisions. This approach enhances the effectiveness of preventive measures and ensures that resources are allocated to areas with the highest risk.
Employee Feedback and Training Gaps
Listening to Your Team’s Concerns
Employee feedback is invaluable in identifying potential issues that may not be immediately apparent through data alone. Encouraging open communication can reveal hidden problems that require preventive action.
Addressing Training Gaps Through CAPA
Training gaps often lead to errors and inefficiencies. A CAPA program should include measures to assess and address these gaps, ensuring that all team members are adequately trained and equipped to maintain quality standards.
Evaluating Supplier Performance
The Impact of Supplier Quality on Your Processes
Suppliers play a crucial role in your supply chain, and their performance directly affects your product quality. Monitoring supplier performance helps identify issues that may necessitate preventive action.
Integrating Supplier Audits into Your CAPA Program
Regular supplier audits as part of your CAPA program ensure that suppliers adhere to your quality standards.
Regulatory Compliance and Audits
Staying Ahead of Regulatory Requirements
Regulatory requirements are constantly evolving, and staying compliant is essential to avoid penalties and ensure market access. Regularly reviewing and updating your CAPA program helps maintain compliance.
Preparing for Audits with a Robust CAPA Program
A robust CAPA program streamlines the audit process by providing clear documentation and evidence of continuous improvement efforts. This preparation minimizes audit findings and demonstrates your commitment to quality.
Leveraging Technology for Effective CAPA
The Role of Advanced QMS in CAPA Implementation
Advanced Quality Management Systems (QMS) facilitate the effective implementation of CAPA programs by automating processes, enhancing data accuracy, and providing real-time insights.
Benefits of Integrating ComplianceQuest for CAPA Management
ComplianceQuest offers a comprehensive platform for managing CAPA programs, change control, and other quality processes. Its seamless integration with existing systems ensures that your team can efficiently identify and address issues, maintaining high standards of quality and compliance.
Preventive Action as a Competitive Advantage
Enhancing Operational Efficiency Through Preventive Measures
Implementing preventive action improves operational efficiency by reducing downtime, minimizing waste, and optimizing resource utilization.
Building a Culture of Continuous Improvement
A culture that prioritizes preventive action fosters continuous improvement. Encouraging proactive problem-solving and innovation ensures that your organization remains agile and resilient in a competitive market.
Conclusion
In an era where regulatory landscapes are becoming increasingly complex, and market competition is fiercer than ever, having a robust CAPA program is essential for sustaining business success. ComplianceQuest stands out as a critical tool for businesses in 2024, offering advanced capabilities for managing CAPA, change control, and overall quality management. By leveraging ComplianceQuest, organizations can ensure seamless compliance, enhance operational efficiency, and foster a culture of continuous improvement. Investing in such a comprehensive solution not only mitigates risks but also empowers your team to proactively address challenges, ensuring long-term growth and stability in the dynamic industries of Life Sciences and Manufacturing.
This post was created with our nice and easy submission form. Create your post!